- Home
- PRODUCTS
PRODUCTS

Orthopedic Implant, Surgical Instrument and Sterile Container Manufacturer, Aysam
Production of Orthopedic Implants, Surgical Instruments and Sterile Containers is a very specific field and companies wishing to operate in this field must meet a number of legal and regulatory requirements. For this reason, a company that wants to operate in this field must first operate in accordance with local legal regulations and quality standards, and then in accordance with the legal regulations and quality standards of the countries it exports to.
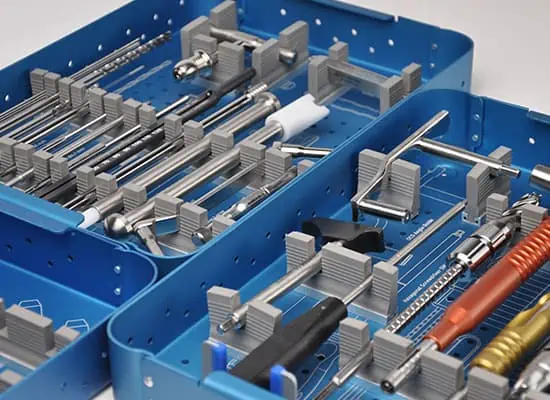
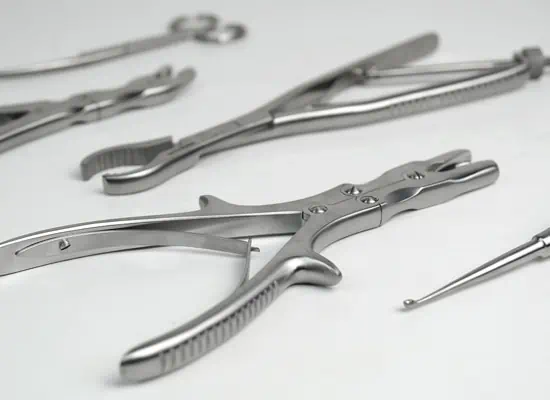
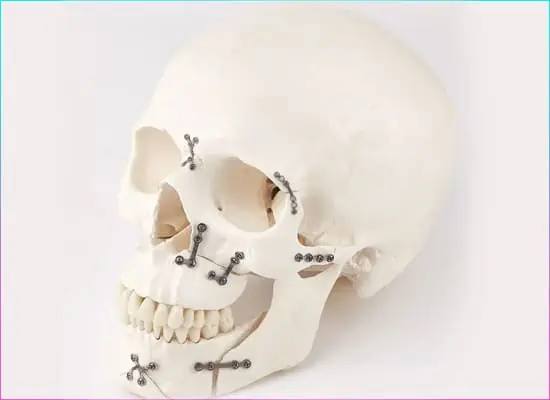
Orthopedic Implant, Surgical Instrument and Sterile Container are designed and produced with different features depending on their intended use. Therefore, first of all, it is necessary to decide which types of products will be produced in accordance with which usage purposes. After this decision, the design process is handled, the necessary verifications are made, the prototypes are produced, the processes are matured for the mass production process, and the mass production begins after the necessary equipment and raw materials are procured. These raw materials include medical grade titanium, stainless steel, CoCrMo (Cobalt Chrome Molybdenum), aluminum and similiar metal alloys, polyethylene, polymers, sterilization filters, sterilization indicators, and mechanical and electronic components.
The manufacturing process consists of a series of steps, starting from product design, to prototype production and mass production of the final product. This process also includes steps such as quality control, testing and determination of conformity of products.
In addition, manufacturers of Orthopedic Implants, Surgical Instruments, and Sterile Containers are required to fulfill legal requirements such as CE (European Compliance), and FDA (American Food and Drug Administration) approval, which are required for their products to be put on the market. Therefore, the company may need to go through a series of specialized steps for legal advisory services and CE and FDA approval.
In addition to all these, Orthopedic Implant, Surgical Instrument, and Sterile Container production is a very competitive field . To be successful, good design, production, management activities, sufficient knowledge, and a good marketing strategy are required. The effective promotion and distribution of products to their target markets is a critical factor in the success of the company.
For these reasons, the production of Orthopedic Implants, Surgical Instruments, and Sterile Containers is a very complex business area. However, with appropriate legal, technical, and marketing support, it is possible to be successful in this field.